|
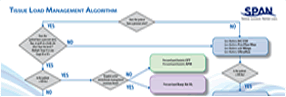
Group 2 code Applies to PressureGuard CFT Description: Code E0373 describes an advanced, non-powered pressure reducing mattress which is characterized by all of the following:
Coverage Criteria: A group II support surface may be covered if the patient meets the following medical criteria:
Documentation:
Medical Policy - Description of EO373 - more information can be found at |
Accessible Navigation
Section Navigation
Search Our Website
Main Navigation
- Products
- Surfaces
- Beds
- Lifts & Slings
- Seating
- Positioners
- Skin Care
- Fall Protection
- Furniture
- About Us
- Clinical/Education
- Videos
- Contact
Footer Navigation
70 Commerce Center
Greenville, SC 29615
800-888-6752
864-288-8692 fax
www.spanbackup.com
©2024 Span America. All rights reserved.
View Accesskeys
Accesskeys
This site contains a number of keyboard shortcuts, called "accesskeys," to assist in navigating. Each Internet browser has a different method of accessing these keys:
- Internet Explorer on a PC use ALT+Accesskey
- Firefox on PC use ALT+SHIFT+Accesskey
- Firefox and Safari on Apple or Linux use CTRL+Accesskey
Please note: Internet Explorer users may also need to hit the “enter” key to activate a link. Apple users with Spaces enabled may need to use the accesskey for Skip to the main navigation (A); then, TAB through the navigation and press "enter" to activate a link.
Page navigation
- 0 : Back to Home
- 1 : Products
- 2 : About Us
- 3 : Clinical/Education
- 4 : Videos
- 5 : Contact
Page jumping
- ? : View Accesskeys
- A : Skip to the main navigation
- C : Skip to the content
- S : Skip to the side bar